
Navigating the Value-Based Care Shift: Key Challenges for Payers (Part 2)
Examining emerging value-based care models to learn how payers and manufacturers are successfully implementing value-based contracts and payment approaches.

This two-part series explores the shift from volume-based to value-based contracts (VBCs) in healthcare, with a focus on specialty drugs and cell and gene therapies. In Part 1, we introduced some of the key concepts and challenges payers and manufacturers face in the evolving healthcare landscape. Part 2 expands on these insights, examining value-based care models and associated payment approaches, highlighting real-world examples of payers and manufacturers successfully implementing these innovative, performance-based strategies.
How Value-Based Care Models Are Impacting Payment Approaches (Continued)
As the U.S. healthcare system increasingly embraces value-based care models, payers and biopharmaceutical manufacturers are also shifting toward more widespread use of value-based contracts (VBCs). These agreements tie healthcare compensation to quality outcomes and cost efficiency rather than volume, and precisely align patient benefits to ensure payment is bound to treatment success. Below are some of the most influential models driving this transformation:
Value-based purchasing: This model ties payment for a medical intervention or manufacturer product to pre-defined performance metrics or payment milestones, rewarding decisions that improve patient outcomes or other real-world results. By incorporating safeguards that promote higher-quality care, value-based purchasing ensures healthcare investments deliver improved results for patients.
Outcomes-based contract: These types of agreements, between a payer and manufacturer, link payment to a treatment’s ability to achieve expected patient health outcomes or other real-world results over a specified period. By tying financial responsibility to effectiveness, this model promotes accountability, incentivizing manufacturers to develop and deliver higher-quality therapies.
Annuity-based contracts with outcomes: A multi-year reimbursement model where payments are distributed over a period of time based on predefined clinical or cost-related outcomes. This structure aligns financial incentives with long-term treatment effectiveness, making high-cost therapies more financially sustainable for payers.
Subscription models: This payment approach provides medical interventions or manufactured products for a fixed fee, either in relation to a specific proportion of patients or on a per-patient basis. Subscription models offer predictable costs, enhance patient access and help manufacturers stabilize revenue and manage financial risk more effectively.
Warranty models: Often managed by a third-party administrator, these policies reimburse treatment-related costs when a product or intervention fails to meet expected outcomes. This approach mitigates financial risk for payers and provides reassurance to patients and healthcare providers about the effectiveness of the treatment, ultimately fostering greater trust in newer therapies.
Annuity-based models: A multi-year contract model that distributes reimbursement installments for manufactured products over an extended period. By spreading payments over time, these models align costs with the long-term value of the treatment, making them more manageable for payers while ensuring sustained access to specialty therapies for patients.
Real-World Applications of Value-Based Contracts (VBCs)
As high-cost therapies become more prevalent, VBCs are being utilized to balance financial sustainability with patient access. Here are some notable real-world examples of their implementation:
Lenmeldy: World’s most expensive drug, a one-time gene treatment for metachromatic leukodystrophy (MLD) that adds a missing gene to the bone marrow cells of children, reversing the condition’s root cause in the brain.
- Cost: $4.25m
- VBCs: Manufacturer offers innovative outcomes- and value-based agreements to both private and government insurers to ensure broad, expedient and sustainable reimbursed access.
Casgevy: First cell-based gene therapy employing CRISPR-based gene-editing technology for treating sickle cell disease (SCD) in patients ages 12 and older who have recurrent vaso-occlusive crises (VOCs). Approved first in the UK, followed by FDA.
- Cost: $2.2m
- VBCs: CMS oversees the Medicaid program and introduced a pilot “access model” for expensive CGTs designating sickle cell as its initial focus. CMS will negotiate an “outcomes-based agreement” that links payment for a drug to the health benefit it delivers. In sickle cell, for example, the targeted outcomes could be continued elimination of pain crises over time.
Lyfgenia: Another cell-based gene therapy for SCD patients ages 12 and older with a history of vaso-occlusive events.
- Cost: $3.1m
- VBCs: CMS oversees the Medicaid program and introduced a pilot “access model” for expensive CGTs designating sickle cell as its initial focus. CMS will negotiate an “outcomes-based agreement” that links payment for a drug to the health benefit it delivers. In sickle cell, for example, the targeted outcomes could be continued elimination of pain crises over time.
Hemgenix: First gene therapy, one-time treatment for adults with moderate to severe bleeding disorder hemophilia B.
- Cost: $3.5m
- VBCs: Manufacturer anticipates discounts, including value-based agreements with commercial payers.
Zolgensma: A one-time gene therapy treatment for spinal muscular atrophy (SMA) in children under the age of two.
- Cost: $2.32m
- VBCs: Manufacturer will allow payments over five years, at $425,000 per year, and will offer partial rebates if the treatment does not produce the expected results.
Xalkori: For treatment of non-small lung cancer.
- Cost: $20,000 for 30-day supply
- VBCs: Manufacturer offers a Pledge Warranty Program in the US. Provides for a rebate when a patient discontinues Xalkori before the fourth refill. It is available to both commercial insurers and Medicare Part D plans. Patients can apply for a rebate for their out-of-pocket costs directly on the manufacturer portal. Strategy is used to address the Medicare best price requirement with a third party managing the program.
Roctavian: A one-time gene therapy used for the treatment of adults with severe hemophilia A.
- Cost: $2.9m
- VBCs: In the United States, the manufacturer is offering to enter into value-based agreements that will cover the companion diagnostic testing and pays refund based on the offset costs of avoiding Factor VIII costs.
These applications illustrate how VBCs are helping payers manage the financial impact of high-cost therapies while ensuring continued access to breakthrough treatments.
The Growing Role of VBCs Within Value-Based Care Models
As VBCs become increasingly integrated within value-based care models, they offer several key advantages for payers and stakeholders:
Reduced Financial Risk – By tying payment to clinical outcomes VBCs help mitigate the financial uncertainty of high-cost treatments.
Improved Real-World Health Outcomes – VBCs ensure that healthcare dollars are efficiently allocated to treatments, reducing wasteful spending and improving healthcare experiences.
Focus on Patient-Centered Care – By aligning incentives with patient needs and preferences, VBCs promote more personalized and effective treatment strategies.
Greater Transparency – VBCs encourage data sharing between stakeholders, enhancing accountability in treatment performance and cost-effectiveness.
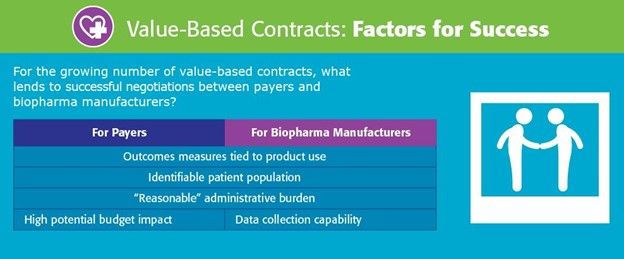 Source: 2024 National Pharmaceutical Council
https://www.npcnow.org/topics/alternative-payment-models/value-based-contracts
Source: 2024 National Pharmaceutical Council
https://www.npcnow.org/topics/alternative-payment-models/value-based-contracts
The bottom line: VBCs help greatly reduce overall healthcare expenses. By prioritizing preventive care and focusing on quality instead of volume, VBCs aim to create a more sustainable and efficient healthcare ecosystem.
The Future of Value-Based Contracts
Looking ahead, the use of value-based contracts is expected to continue to increase, driven by:
- Advanced Data and Analytics - Improved tracking of patient outcomes and drug performance.
- Shifting Healthcare Incentives - Increased participation in Accountable Care Organizations (ACOs) and alternative payment agreements will further enhance value-based care models.
As these trends continue, VBCs are poised to have a pivotal role in shaping the future of healthcare —promoting accountability, fostering collaboration, and improving patient care.
AscellaHealth: A Trusted Partner
AscellaHealth serves as a trusted partner that effectively aligns the interests of both payers and manufacturers. As payers strive to meet the unmet treatment needs of individuals with complex, chronic conditions and rare diseases, they turn to our experienced team for guidance in navigating the complexities of VBCs, including data collection, evidence development, suitable outcome measures, implementation costs, and effective financial incentives. By collaborating with AscellaHealth, payers are better equipped to implement cost-effective approaches that enhance patient care and optimize treatment results.
To learn how AscellaHealth can assist your organization in more efficiently managing specialty pharmaceutical therapies and mitigating financial risks, please click here or contact us directly at businessdevelopment@ascellahealth.com.
